In 2019, spectacular “chalcopyrite” specimens, consisting of clusters of iridescent spheres, appeared on the collector mineral market. The reported source was the Tonglushan Copper Mine, near Daye, Hubei Province, China. The Smithsonian Institution acquired one of these specimens at the 2019 Tucson Gem and Mineral Show.

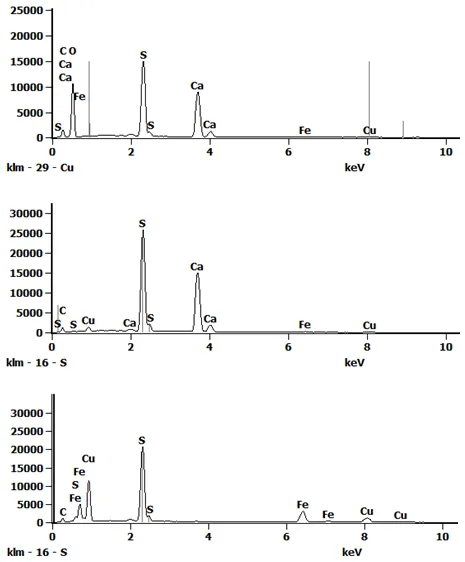
Preliminary analytical work on some of the spheres was done at the University of Arizona and confirmed the composition as copper iron sulfide by energy-dispersive x-ray spectroscopy (EDS). In addition, Stuart Mills at Museum Victoria also identified chalcopyrite and pyrrhotite by x-ray diffraction. To further characterize these unusual specimens, an investigation was undertaken on sample surfaces and polished cross sections from the Smithsonian’s specimen in the Department of Mineral Sciences.
Our preliminary EDS point analysis of spheres within a polished section also confirmed the presence of copper iron sulfide but unfortunately, the global pandemic halted further study. When the museum was reopened to staff, a new field emission analytical SEM was available in the department of Mineral Sciences. The polished section of the Smithsonian’s sphere was left exposed to the air for many months and the sample had grown crystals of calcium sulfate on the surface. This prompted a more in-depth investigation utilizing the department’s new analytical scanning electron microscope.

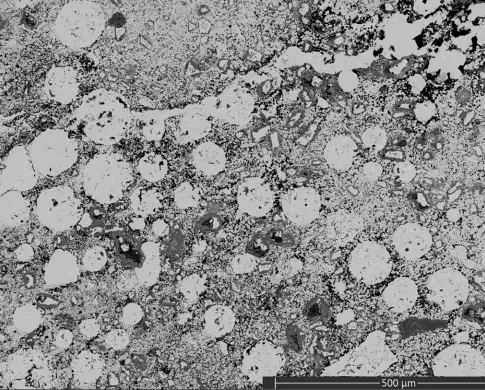
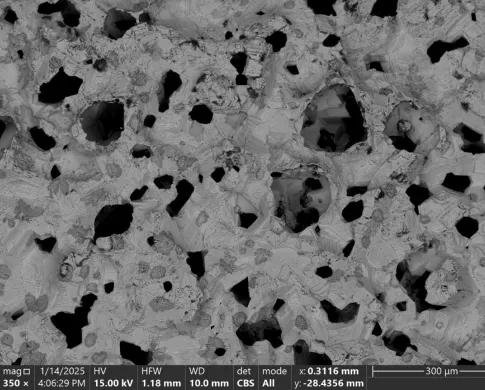
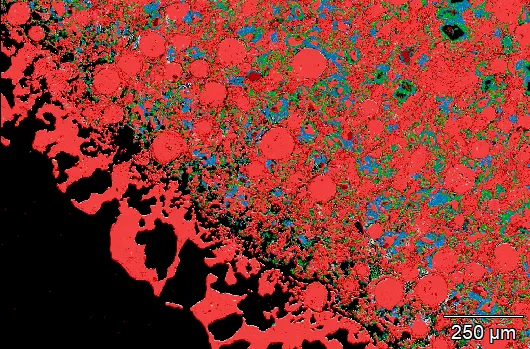
The sample was repolished before imaging and analysis. Interesting textures and phases were revealed within the spheres. The spheres have a more porous chalcopyrite rim with an interior containing regions of 10-100 micron copper iron sulfide spherules in a finer matrix. These regions varied in density as you moved toward the interior.

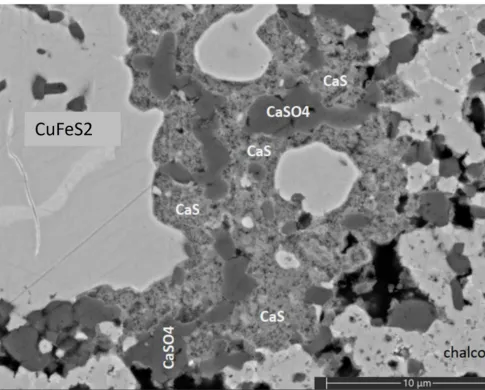
Elemental mapping showed areas of copper iron sulfide with interstices containing calcium sulfate and curiously, calcium sulfide! Other minor phases encountered include quartz, muscovite, and sodium feldspar.
|
|
CaS (wt. %) |
CaS (atom %) |
CuFeS2 (wt. %) |
CuFeS2 (atom %) |
CaSO4 (wt. %) |
CaSO4 (atom %) |
|
Ca |
56.28 |
50.74 |
|
|
29.12 |
16.5 |
|
Cu |
|
|
35.28 |
25.74 |
|
|
|
Fe |
|
|
31.43 |
26.1 |
|
|
|
S |
43.72 |
49.26 |
33.29 |
48.16 |
24.02 |
17.01 |
|
O |
|
|
|
|
46.85 |
66.49 |
Of all the phases noted during the investigation, the calcium sulfide was particularly interesting as this phase is commonly found in meteorites but is only found from a few natural localities with exotic conditions such as a burned coal seam around the Dead Sea.
Raman spectroscopy was utilized to verify chalcopyrite and anhydrite in the sphere sections. While Raman was useful in identifying these two major phases, calcium sulfide is not Raman active and could not be verified with this method.
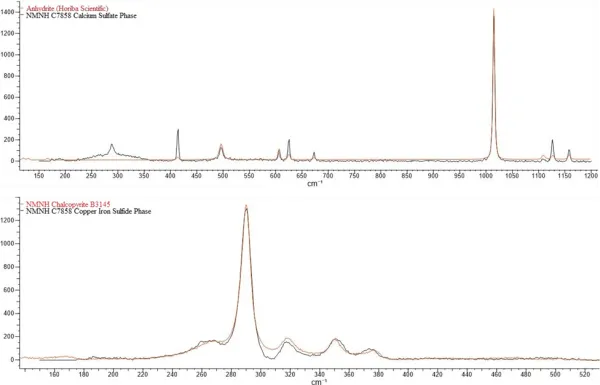
Multiple sections were made and analyzed from the Smithsonian’s specimen. Because of small size and stability of the calcium sulfide phase, we were unable to perform x-ray diffraction (XRD) analysis to confirm the actual calcium sulfide mineral. The Department of Mineral Sciences is in the process of adding a new XRD instrument with different capabilities that may enable us to map the section without attempting to powder the sample or extract such a tiny portion of the phase.